Operation Warped Science Part 4
The Army, the JPEO and how they created National Resilience
Let’s take a deeper look at the biological weapons history behind Resilience, the company that produced Moderna’s Covid-19 jabs and you can decide for yourselves what is really going on.
Ology/Nanotherapeutics was contracted by the US Government to develop countermeasures for Ebola, COVID-19, anthrax, botulinum toxin, and smallpox. More specifically, the DoD ADM facility, operated by Ology Bioservices, has been involved in the production of an anti-Ebola monoclonal antibody (mAb114). This effort was a collaboration with the Vaccine Research Center at the National Institutes of Health, funded in part by DARPA. The production included technology transfer and cGMP manufacturing to ensure rapid and efficient delivery of this critical countermeasure (Sources: BioSpace &JPEO CBRND).
The DoD’s ADM has developed countermeasures against anthrax, including vaccines and antitoxins, to protect military personnel and the public from potential bioterrorism threats involving Bacillus anthracis [Anthrax.]Years prior to the pandemic their efforts already included developing monoclonal antibodies and vaccines for threats like Botulinum neurotoxin injectables for our warfighters and other potential bio-weapons (Sources: JPEO, Pharmaceutical Online & Global Biodefense).
A broader look into the BWs they were working on at Ology is detailed briefly here:
Ology Bioservices was awarded a contract by the Biomedical Advanced Research and Development Authority (BARDA) to develop and manufacture a monoclonal antibody-based vaccine against Ebola virus. The vaccine, known as MB-003, was developed in collaboration with the National Institutes of Health (NIH) and is designed to provide protection against multiple strains of Ebola virus. Anthrax vaccine: Ology Bioservices has been involved in the development and production of a next-generation anthrax vaccine, known as AV7909. The vaccine is being developed in collaboration with Emergent BioSolutions [who received over 900M in OWS] and is designed to provide protection against inhalational anthrax.
Furthermore, Ology Bioservices is collaborating with the Public Health Agency of Canada (PHAC) and Providence Therapeutics to produce a COVID-19 vaccine. The vaccine, known as PTX-COVID19-B, is based on messenger RNA (mRNA) technology and is designed to provide protection against the SARS-CoV-2 virus.
BARDA Contract: Ology Bioservices was awarded a contract by BARDA worth up to $628 million to provide advanced development and manufacturing services for medical countermeasures against biological and chemical threats.
NIH Grant: Ology Bioservices received a $10 million grant from the NIH to support the development of a next-generation anthrax vaccine.
DoD Contract: Ology Bioservices was awarded a $24.5 million contract by the Department of Defense (DoD) to develop and manufacture medical countermeasures against chemical and biological threats.
**The number of contracts and BW countermeasures produced are many and this list was by no means an exhaustive or definitive list.
Although the JPEO-CBRND is the official parent of the ADM and its sister program ADAMANT-PRISM, it is important to note how the Defense Advanced Research Projects Agency (DARPA) also played a significant role in the broader context of advanced development and manufacturing, particularly through initiatives aimed at accelerating biomedical research and developing innovative technologies for rapid response to biological threats. DARPA helped ensure that the United States was prepared to quickly and effectively respond to biological threats. The ADM facilities, in turn, provided the necessary infrastructure to scale up and produce these critical medical countermeasures.
It was DARPA who provided funding for early-stage research and development of medical countermeasures, namely their 2013 contract with Moderna. Keep in mind, that at this point in time, Moderna had NEVER developed a marketable FDA approved product and the company itself was only 3 years old. This begs the question, why would our government [DARPA] invest tens of millions of taxpayers’ dollars into a new company that had no portfolio of products that would suggest they were the best candidate for the job? We can only speculate but the coincidental timing of their contract with Moderna in 2013 should not be dismissed. I say a coincidence because certainly the fact that the Army’s JPEO-CBRND started it’s ADM program with Nanotherapeutics in 2013 the very same year that DARPA started its multi-million-dollar contract with Moderna was nothing more than by chance.
How lucky for the DOD to have the monopoly on mass-scale rapid development of medical countermeasures and be first in line to create mRNA countermeasures with Moderna. What was even more fortunate was how lucky Moderna was! Moderna gets a 25+ million-dollar contract with the DoD [DARPA] after just 3 years of existence and they didn’t even need a product to sell. Moreover, imagine just how fortunate Moderna must feel that when the pandemic arrived that they would be among the few companies contracted through OWS & manage to land one of the largest medical contracts in history. Especially considering that they’d get that elite contract despite not being actually capable of fulfilling because lucky them, a “new” heavily funded advanced manufacturing company is going to help you because they just so happen to have all the biological countermeasure history, the facilities and the government funding to complete the job for them! This history behind the JPEO’s ADM and DARPA’s P3 program laid the groundwork the government’s ability for total vaccine control as well as give them access to the ADM /Ology BSL-3 laboratory in Alachua, Fl without having to deal with prying eyes.
DARPA’s Role
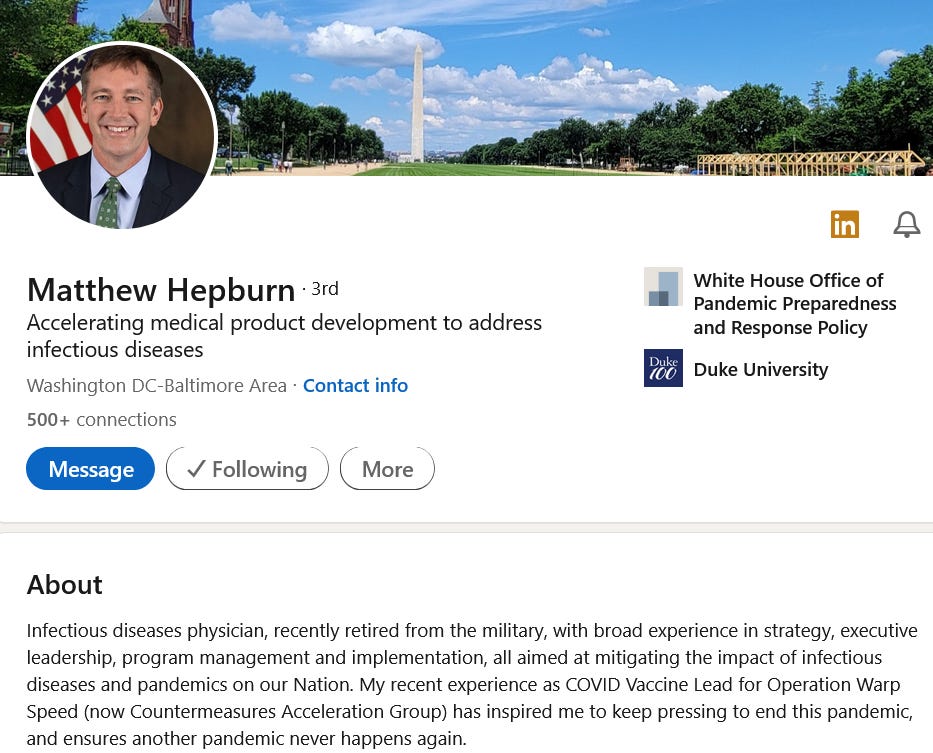
DARPA’s programs such as the Biological Technologies Office (BTO) were already focused on developing new technologies for detecting, diagnosing, and treating infectious diseases. Programs such as the ADEPT-PROTECT program & the Pandemic Prevention Platform (P3). Both these platforms aimed to create a platform that can develop and deploy medical countermeasures within 60 days of an outbreak. The platforms most sought after by ADEPT and P3 were mRNA “vaccines” and monoclonal antibodies These initiatives involved the development of rapid vaccine production technologies, some of which can be transitioned to production at ADM facilities. This entire program which was spearheaded by DARPA program manager, Matthew Hepburn who was the lead pioneer for DARPA’s interest into RNA vaccine technology, which played a crucial role in the development of COVID-19 vaccines.
A quick look into who Matthew Hepburn is: In 2010, Matthew Hepburn [Col. Ret] joined DARPA. He spent the first few years focused on innovative medical platforms, namely monoclonal antibodies, and mRNA. Hepburn is responsible for founding and leading the ADEPT PROTECT project. Additionally, he is currently the CMO of the Joint Program Executive Office for CBRN [JPEO-CBRN]. Before managing to secure that high position Hepburn was the Vaccine Development Lead for the Countermeasures Acceleration Group [CAG] -formerly known as Operation Warp Speed [May 2020].
Hepburn’s background stems from a 23yr career in the US ARMY as well as his time being the Director of Medical Preparedness on the White House National Security Staff [2010-2013], Clinical Research Director at USAMRIID [2007-2009] -leading domestic clinical research efforts on bio-defense products and his six years as Program Manager at DARPA [2013-2019]. Interestingly Hepburn attended his undergrad at Duke University in North Carolina.
Now that we know Hepburn a little bit better we can turn our attention back to his role in all of this. The manufacturing capabilities supported by ADM facilities, and Hepburn’s programs at DARPA were absolutely essential for the creation and scaling up these RNA “vaccines” for Moderna.
DARPA’s investments in advanced manufacturing techniques, such as continuous manufacturing and modular production systems, contribute to the capabilities of ADM facilities to produce medical countermeasures efficiently and at scale and the synergy between DARPA’s innovative research programs and the operational capabilities of ADM facilities ensures that breakthroughs needed in medical countermeasures was strategically precisely placed action for what the government was seeking, a means to get the untested countermeasures through their Other Transactional Agreement’s while being able to scale up the production in a war-time manner.
The public private partnership that developed was really just a means to exploit loopholes in the biological weapons convention and obfuscate regulatory manufacturing policies. We see examples of this when in February 2022, Resilience/Ology landed a major Contract Award with the DoD, a contract worth up to $250 million to develop and manufacture a monoclonal antibody treatment for botulinum neurotoxins (BoNT). This ADM's Monoclonal Antibodies (mAbs) for Botulism Neurotoxin (BoNT) had long been a primary goal of the Army. In this instance the ADM facility developed and manufactured mAbs to support Phase 1 clinical trials against Botulism Neurotoxin. This included submitting an Investigational New Drug (IND) application to the FDA, which was a significant milestone in the regulatory process.
In fact, the COVID-19 countermeasure that Resilience made for Moderna, to my knowledge, is the first and only countermeasure they’ve created for civilian use. The pandemic provided everything that DoD, the JPEO-CBRN and DARPA had spent decades aspiring and countless millions for had now, not only become possible, but achievable and they were able to do so without public outcry or legislative push-back.
Operation Warp Speed: A Modern Parallel
Operation Warp Speed, a public-private partnership initiated in May of 2020, was the demonstration that the ADM’s expedited processes and the strategic collaborations were successful and conducive to the nation’s bio-defense efforts. This initiative aimed its operations to accelerate the development, manufacturing, and distribution medical countermeasures in a military like fashion- demonstrating the evolution of medicine and public health.
This convergence of powers and abilities ran by the government is precisely what 35th President John Fitzgerald Kennedy had warned about in April of 1961. The fear that emergencies would be declared in order to usher in this type of control was seen when he said that there was a “very grave danger that an announced need for increased security will be seized upon by those anxious to expand its meaning to the very limits of official censorship and concealment.” That this would be done by a entity that would wield concerted power one that he claims is “a system which has conscripted vast human and material resources into the building of a tightly knit, highly efficient machine that combines military, diplomatic, intelligence, economic, scientific and political operations.”
This system, one in the hands of an unhinged Uncle Sam while under the veil of secrecy quickly becomes a reckless and deceptive paramilitary approach to managing biological threats.
**This concludes the 4 part series on Operation Warped Science.
Links to the sources for the series which aren’t embedded hyperlinks are found here https://www.one-tab.com/page/iBC3xSwNShSlbQ2iKw5oYw











In Europe Lonza produced for Moderna. You didn't mention Lonza.
https://baselarea.swiss/de/blog-post/lonza-stellt-corona-impfstoff-fuer-moderna-her/
https://www.chemanager-online.com/news/lonza-und-moderna-gehen-weltweite-strategische-zusammenarbeit-ein
https://www.bloomberg.com/news/articles/2020-11-19/moderna-vaccine-production-is-gearing-up-partner-lonza-says
No military partnership in that cooperation, I guess.
no there is, but lonza only did fill and finish not formulation. It would like me including the shipping process...